Key Takeaways
- Detectable ctDNA indicates minimal residual disease and increased recurrence risk in colorectal cancer patients post-curative therapy.
- The phase 2 trial investigates AMB-05X, targeting CSF1R-positive macrophages, in ctDNA-positive CRC patients.
- The trial design includes a single-arm, open-label approach, enrolling patients with no measurable radiographic disease post-standard therapy.
- Primary endpoint is ctDNA clearance rate, with exploratory endpoints including survival rates and pharmacokinetic profiles.
- The trial, initiated in December 2024 at MD Anderson Cancer Center, is expected to complete in February 2028.
Detectable circulating tumor DNA (ctDNA) is a surrogate marker for the presence of minimal residual disease (MRD) and is associated with an increased risk of disease recurrence after curative-intent therapy in patients with colorectal cancer (CRC).1 However, the optimal treatment for patients with detectable ctDNA without measurable radiographic disease after completion of standard-of-care (SOC) therapy has yet to be determined. A phase 2 study is seeking to expand this treatment paradigm by investigating the CSF1R-directed antibody AMB-05X in patients with ctDNA-positive CRC.
What was the rationale for investigating AMB-05X in patients with CRC with detectable ctDNA?
Previously, researchers at The University of Texas MD Anderson Cancer Center in Houston identified the presence of CSF1R-positive, pro-oncogenic macrophages in murine models with CRC micrometastases. In vivo, blocking CSF1R with an anti-CSF1R antibody was shown to reduce levels of pro-tumorigenic micrometastases and CD163-positive macrophages in mice with MRD, as well as prevent the development of CRC macrometastases.
What is the design of the phase 2 trial evaluating AMB-05X in ctDNA-positive CRC?
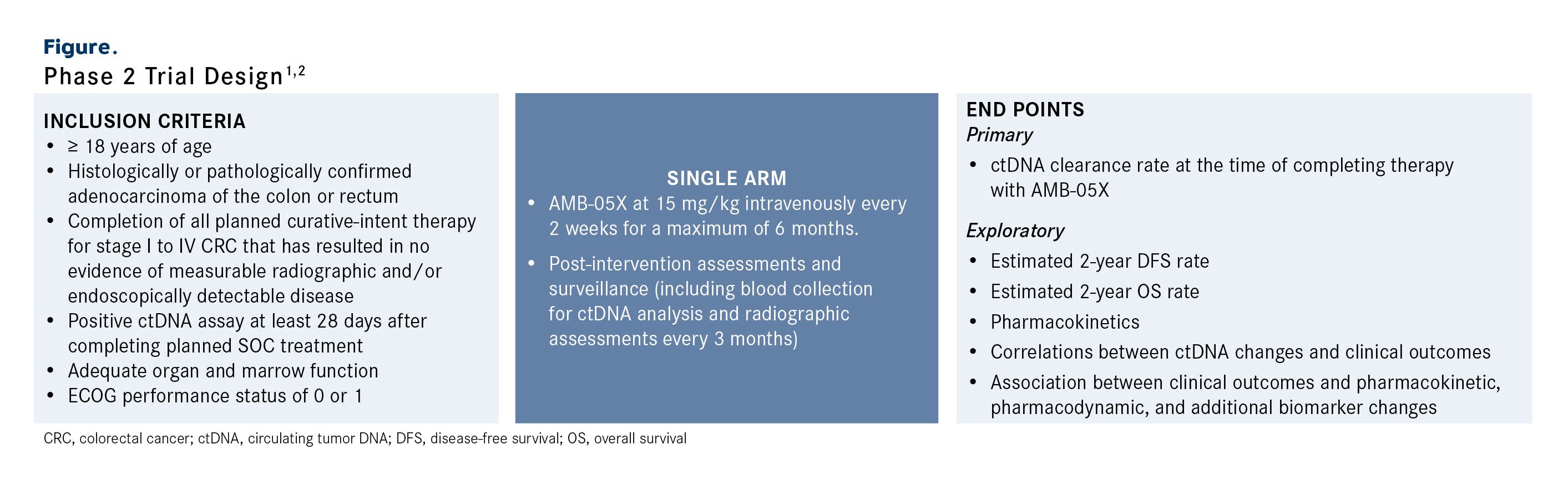
This investigator-initiated, prospective, single-arm, open-label phase 2 trial is enrolling patients at least 18 years of age with histologically or pathologically confirmed adenocarcinoma of the colon or rectum (Figure).2 Patients must have completed all planned curative-intent therapy, including chemotherapy and surgery, if indicated, for stage I to IV CRC that has resulted in no evidence of measurable radiographic disease per RECIST 1.1 criteria and/or clinically detectable disease at least 28 days after completion of planned SOC treatment.1,2 Patients are also required to have a positive ctDNA assay at least 28 days after completing planned SOC treatment, adequate organ and marrow function, and an ECOG performance status of 0 or 1.2
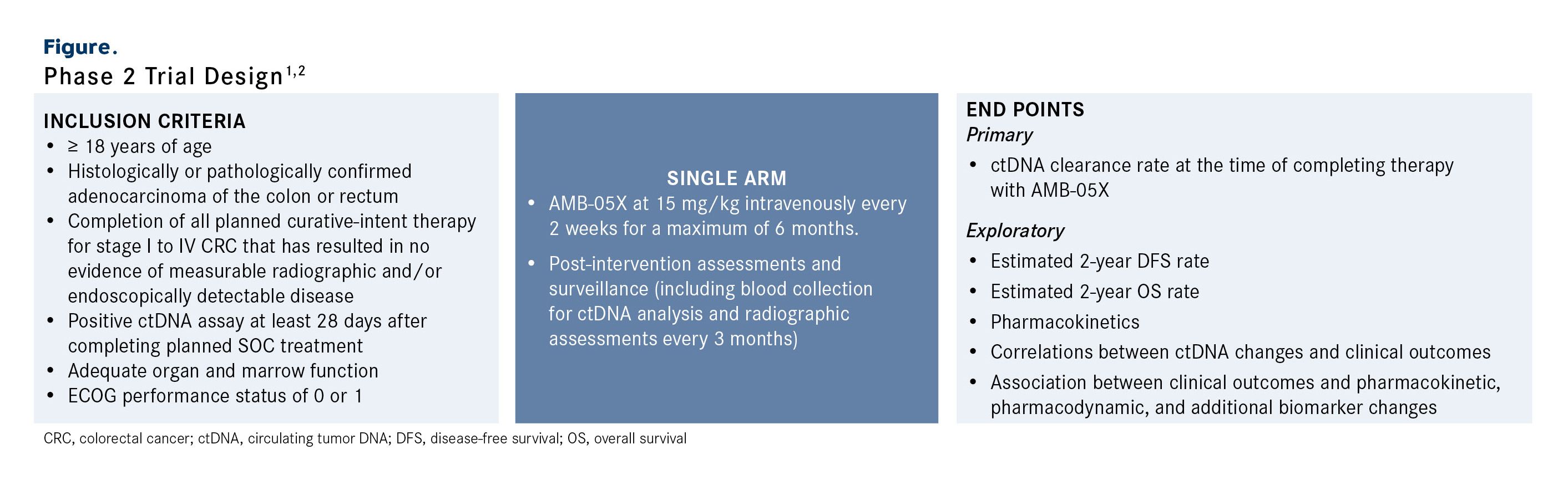
Figure. Phase 2 Trial Design1,2
Patients will be excluded if they have or have had a prior or concurrent malignancy within 3 years of trial registration for which the natural history of treatment may interfere with the safety or efficacy assessment of AMB-05X or which requires concurrent therapy; clinically significant hepatobiliary disease that would lead to excessive treatment risk during the study; significant concomitant health conditions; persistent adverse effects related to prior anticancer therapy that are at least grade 2 in severity; and a co-existing separate disease, metabolic disorder, clinically significant laboratory result, or any other condition that may prohibit the use of AMB-05X or put the patient at undue risk of harm.
Patients in this single-arm trial will receive AMB-05X at 15 mg/kg intravenously every 2 weeks for a maximum of 6 months.1 Following treatment, patients will undergo post-intervention assessments and surveillance; this includes blood collection for ctDNA analysis and radiographic assessments (CT or MRI) conducted every 3 months (± 14 days). The trial plans to enroll 15 total patients. As of October 7, 2025, 10 patients have been dosed.
The primary end point is identification of the ctDNA clearance rate at the time of completing therapy with AMB-05X. Exploratory end points include the estimated 2-year disease-free survival and overall survival rates; the pharmacokinetic profile of AMB-05X; correlations between ctDNA changes and clinical outcomes; and the association between clinical outcomes and pharmacokinetic, pharmacodynamic, and additional biomarker changes.
The trial was initiated On December 4, 2024, and is being conducted at The University of Texas MD Anderson Cancer Center.2 The estimated primary completion date is February 1, 2026, and the trial is expected to run until February 1, 2028. The trial is currently enrolling.
References
- LaPelusa M, Mohamed A, Sun R, et al. Open-label phase II trial of AMB-05X for patients with ctDNA(+) colorectal cancer after curative-intent treatment. Ann Oncol. 2025;36(suppl 2):S593-S594. doi:10.1016/j.annonc.2025.08.1478
- Trial of AMB-05X for patients with ctDNA(+) colorectal cancer after curative-intent treatment. ClinicalTrials.gov. Updated October 30, 2025. Accessed November 4, 2025. https://clinicaltrials.gov/study/NCT06617858

